Visual Vestibular Mismatch
Download PDF version (3.4MB)
Chapter 8
Caloric response does not decline with age
Mallinson AI, Longridge NS.
J Vest Res 2004;14(5):393-396.
ABSTRACT
Complaints of imbalance in the elderly are commonly heard by clinicians, and pathology of the vestibular system may play an important role in these complaints. While there is solid anatomical evidence for age-related decline of some vestibular structures, a corresponding deterioration in physiologic function has not been convincingly demonstrated.
Vestibular function is traditionally measured with caloric irrigations. Although there has been some age dependent change in caloric response shown, there is no good parallel between caloric response and imbalance in the elderly patient.
Our experiment confirms that slow phase velocity of caloric responses does not decline with age. Calorics measure only one part of the vestibular system, and so should not be regarded as representative of balance system function. As a result, measured caloric response does not parallel documented anatomic age-related decline of the vestibular system.
KEYWORDS: calorics,aging,imbalance,otoliths,vestibular
INTRODUCTION
Instability in the elderly is a well documented and widely researched problem. Instability is the result of some dysfunction of balance, and several studies have shown that stability decreases and postural sway increases with age (10,15). However the reasons why aging adversely affects balance are not fully understood (4). Although Lord states (7) that there are no reports of an association between impaired vestibular function and instability in older people, Kristinsdottir et al. (5) state that vestibular dysfunction is fairly common in elderly individuals.
Vestibular deterioration with aging is known to occur in vestibular hair cells (13) and also nerve cells (2). Ross et al in 1976 (14) specifically showed degeneration of the otolith structures themselves. However despite the evidence provided to support anatomical deterioration of these structures, it has been difficult to illustrate a corresponding deterioration in physiologic function (for instance, utilizing the vestibulo-ocular reflex (VOR)) (11). Peterka et al. (12) did show a small decline, on average, in caloric responses with age based on a linear regression analysis, but called the results “ambiguous”.
It has been suggested in the past that perhaps the lack of a correlation between anatomy and physiology may relate to the absence of clinical manifestations of the aging vestibular apparatus (16). Paige has suggested that our measurement techniques may not have “stressed” the VOR sufficiently to overcome the adaptive phenomena that are available to correct VOR performance deficits (11). He has also suggested that progressive anatomical deterioration with age can be viewed as a partial bilateral vestibulopathy (i.e. an age related “lesion”), which suggests that this “deficit” could potentially be measurable.
Later studies have been carried out to show that vestibular function is in fact related to instability and falling. One study (6) showed a very high correlation between fall related hip fractures and unilateral vestibular disease. In that study, patients who had suffered fall related hip fractures had a significantly higher incidence of head shake nystagmus compared to controls (indicating vestibular disease) and 75% of the patients with head shake nystagmus had fallen towards the slow phase of the nystagmus (which is what would be expected if the fall was vestibular related). It has also been suggested that vestibular disease may contribute to general instability in the elderly (5).
The “gold standard” of vestibular measurement is the caloric test as outlined by Barber (1). A caveat is that caloric testing measures only the function of the lateral semicircular canal (SCC). It does not assess the superior SCC, the posterior SCC, or the maculae, and so it is reasonable to question whether caloric testing can be used to quantify the physiologic decrease in function we assume should parallel anatomic age-related deterioration of the vestibular system.
Mulch et al. (8) addressed the question of age-related caloric response and provided an extensive review of work done in this regard. Their only definitive conclusion was that certain parameters of caloric response showed varying age dependent behaviour. However it has been suggested that decline in normal caloric response does not parallel the progressive course and level of deterioration shown in anatomical studies (12). One of the very few studies that measured caloric responses according to the accepted methods outlined by Barber (1) (analyzing maximum slow phase velocity) was conducted by Van der Laan and Oosterveld (16). They showed that responses increased in intensity up to the age of 40, and then progressively decreased with increasing age. Similar results were shown by Mulch and Petermann (8).
As mentioned previously, caloric testing is a measurement of lateral SCC function only, and perhaps should not serve as a valid measurement of age-related balance system dysfunction. In addition, studies reporting SCC deficits related to age are not well detailed in experimental procedure, or subject selection. A previous study to investigate change in caloric response with age was carried out by Peterka et al. (12). In their analysis they used an “average response” (mean of all four calorics). As patients age, there is a greater chance that they may have suffered from unilateral vestibular pathology (i.e. past history of acute vestibulopathy) which could reduce the response on one side, and hence also reduce the “total response” in any subsequent bithermal caloric test. We were concerned that this may have been a confound in studies which do report senescence of SCC function over the age of 40, and that calorics may in fact not be a true reflection of age-related vestibular decline. We proposed an experiment to see whether or not lateral SCC response to caloric testing does decline over age, designing the experiment to minimize any effect of previous vestibular insult.
METHODS
The charts of 185 patients referred sequentially to our clinic for dizziness were examined. This included 88 males and 97 females ranging in age from 9 to 89 years of age. Although our study would now require ethical approval from both the University and the Hospital, approval at the time of this study was not required, as the study fell into the category of a retrospective chart review of patients who had been exposed to only standard clinical assessment in our unit.
In our laboratory, we utilize the protocol outlined by Barber (1) that is used to obtain maximum vestibular response. All caloric testing was performed and analyzed by a single assessor, to eliminate the possibility of assessor variability. We calculated the mean of the hot and cold caloric responses (slow phase velocities) on the better responding side of all 185 charts.
All results of patients with previous history of vestibular complaints prior to their presenting troubles were excluded, as it is possible that a patient with more than one acute vestibular event could have suffered one lesion on each side. Also excluded were patients with any previous exposure to aminoglycosides or other ototoxic medications, and patients who had taken sedative medication in the last 48 hours. To address the concern that we were studying maximum caloric response in patients who had vestibular disease, we took the position that patients with true vertigo by definition have unilateral dysfunction, so by using the higher responding side, we were obtaining data from their nondiseased ear.
When scoring calorics, nystagmus seen in the first 15 seconds of an irrigation is by definition a spontaneous nystagmus (1), and any such record was discarded from the study. Any repeat ENG was also excluded, as repeat calorics can habituate response.
In analyzing one ear only, we felt that we were also cutting by 50% the chance that a patient had suffered a previous asymptomatic vestibular insult to the measured ear, and so a “maximal” response to calorics could be obtained. Although not made entirely clear in the literature, calorics have never to our knowledge been reported in this fashion before.
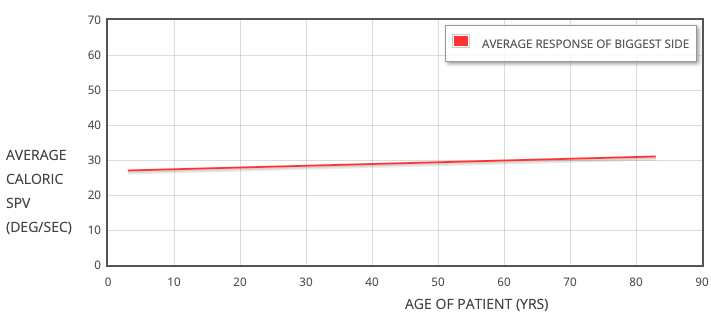
Fig. 1. “Best side average” caloric slow phase velocity plotted against age in 185 patients.
(R2 = 0.0043) (P = 0.37).
RESULTS
We plotted caloric scores against age. We followed the lead of Peterka et al. (12) and looked at “average response” (the mean of the two calorics on the strongest side) and plotted this value against age (Fig. 1). A linear regression was performed to see if a relationship was suggested.
The r2 value for the average of the calorics plotted against age was 0.0043, with a p-value of 0.37. The r2 value suggests that only 0.43% of the variation in caloric score can be explained by age. The very high p-value prevents us from suggesting there is any slope to the line.
DISCUSSION
Regardless of the actual mechanism, one of the abilities that is compromised in the elderly is the ability to control eye movements and foveate images. This is suggested by Paige (11) and also by Peterka et al. (12), both of whom measured VOR deficits related to age. Mulder et al. (9) have also shown that subjects over 70 years of age are impaired in their ability to adapt rapidly to disturbed peripheral conditions, both external and also internal. Perhaps the combination of a slight change in VOR coupled with senescence of the adaptive plasticity mechanisms (i.e. loss of ability of these mechanisms to compensate) plays an important role in the development of age related imbalance.
It has also been suggested that the reason the semicircular canal-ocular responses decline with age is related to a degradation of velocity storage, a hypothesized circuit that reduces phase lead and lengthens the dominant VOR time constant (4). This is probably a multineural signal processing that involves both the vestibular nuclei and also central processing. However, degradation of this hypothesized circuit will result in loss of ability to foveate an image effectively, precluding the ability to maintain balance (or the ability to compensate for a vestibular deficiency).
In summary, our findings suggest that caloric responses (i.e. measured semicircular canal-ocular responses) do not reflect anatomically documented age related senescence of the vestibular system. While caloric response remains a crucial (and one of the only) measures of vestibular function, our data and our method of analysis supports the findings of Peterka et al. (12) that age related effects on caloric results were ambiguous. In their analysis, Peterka et al. did find an average decrease with increasing age, but our method of using the highest side in each patient in an attempt to minimize the effects of previously suffered vestibular events may help to minimize the influence of acute attacks on a labyrinth over time (the possibility of which increases with advancing age). In short, caloric testing is clinically important in delineating the presence of a recent or remote unilateral lesion, but should not be regarded as a measure of true equilibrium and stability in the elderly. Aging does adversely affect equilibrium, and for upright balance to be maintained, the otolith organs may provide a critical signal concerning an impending perturbation of that equilibrium (9). Despite the maintenance of the semicircular canal signal, dysfunction of the vestibular system is common in elderly individuals (5). The only method presently described as being capable of measuring age-related otolithic decline is OVAR testing. Furman and Redfern (4) have used OVAR to show an age-related decline in otolith-ocular responses, which is hypothesized to result from a decline in central vestibular processing, rather than from a loss of function of the otoliths themselves. In summary, the caloric responses in our patients do not show an age related decline, and this agrees with Furman and Redfern's statement (4) that the aging peripheral vestibular system remains functionally intact. Perhaps the low stimulus amplitude signal supplied by the caloric test does not challenge the semicircular canal system sufficiently to reveal its defects, and we feel the caloric test should not be used as an indicator of age-related decline of balance system function.
REFERENCES
- H.O. Barber and C.W. Stockwell, Manual of Electronystagmography, (2nd ed.), C.V. Mosby. St. Louis, 1980.
- B. Bergstrom, Morphology of the vestibular nerve; 2: the number of myelinated vestibular nerve fibers in man at various ages, Acta Otolaryngol 76 (1973), 173-179.
- F.O. Black, W.H. Paloski, M.F. Reschke, M. Igarashi, F. Guedry and D.J. Anderson, Disruption of postural readaptation by inertial stimuli following spaceflight, J. Vest. Res. 9(1999), 369-378.
- J.M. Furman and M.S. Redfern, Effect of aging on the otolith-ocular reflex, J. Vest. Res. 11 (2001), 91-103.
- E.K. Kristinsdottir, P.A. Fransson and M. Magnusson, Changes in postural control in healthy elderly subjects are related to vibration sensation, vision and vestibular asymmetry, Acta otolaryngol 121 (2001), 700-706.
- E.K. Kristinsdottir, G.B. Jarnlo and M. Magnusson, Asymmetrical vestibular function in the elderly might be a significant contributor to hip fractures, Scand. J. Rehab. Med. 32 (2000), 56-60.
- S.R. Lord, Physiological factors associated with falls, Plenary Presentation ISPGR 2003, Sydney Australia.
- G. Mulch and W. Petermann, Influence of age on results of vestibular function tests, Review of literature and presentation of caloric test results, Ann. Otol. Rhinol. Laryngol. 88(Suppl 56) (1979), 1-17.
- T.H. Mulder, W. Zijlstra and A. Geurts, Assessment of motor recovery and decline, Gait and Posture 16 (2002), 198-210.
- M.E. Norre, G. Forrez and A. Beckers, Vestibular dysfunction causing instability in aged patients, Acta Otolaryngol 104 (1987), 50-55.
- G.D. Paige, Senescence of human visual-vestibular interactions: vestibulo-ocular reflex and adaptive plasticity with aging, J. Vest. Res. 2 (1992), 133-151.
- R.J. Peterka, F.O. Black and M.B. Schoenhoff, Age-related changes in human vestibulo-ocular reflexes: sinusoidal rotation and caloric tests, J. Vestib. Res. 1 (1990-1991), 49-59.
- U. Rosenhall, Degenerative patterns in the aging human vestibular neuro-epithelia, Acta Otolaryngol 67 (1973), 208-220.
- M.D. Ross, D. Peacor, L.G. Johnsson and L.F. Allard, Observations on normal and degenerating human otoconia, Ann. Otol. Rhinol. Laryngol. 85 (1976), 310-326.
- J.H. Sheldon, The effect of age on the control of the sway, Gerontol. Clin. 5 (1963), 129-138.
- F.L. Van der Laan and W.J. Oosterveld, Age and vestibular function, Aerospace medicine 45 (1974), 540-547.